Introduction
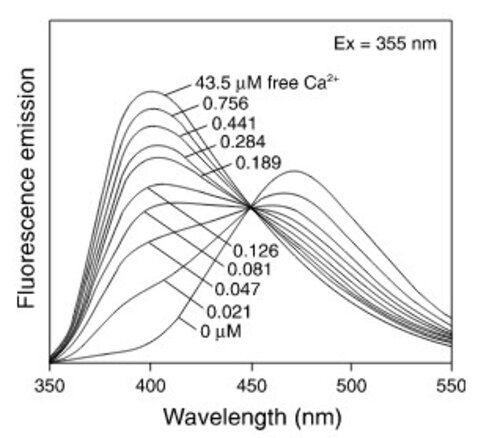
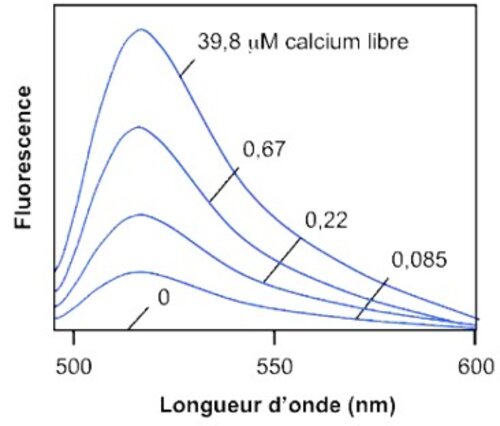
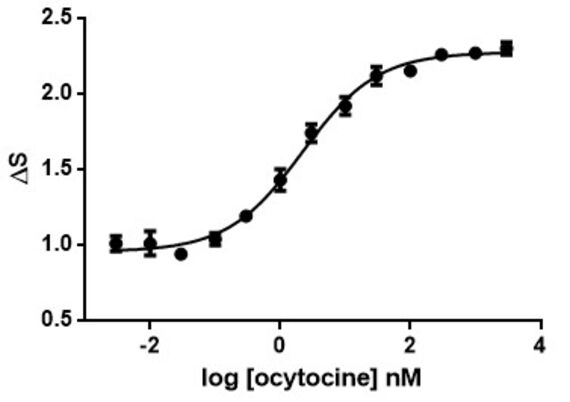
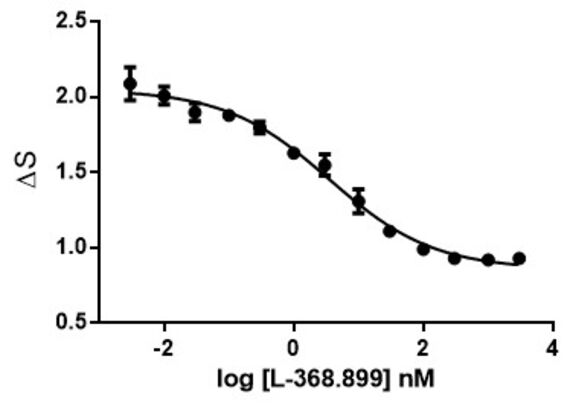
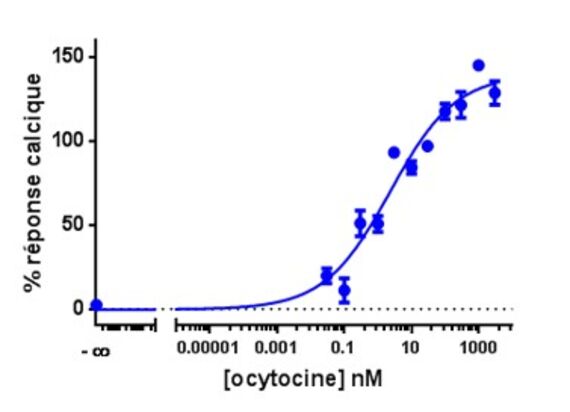
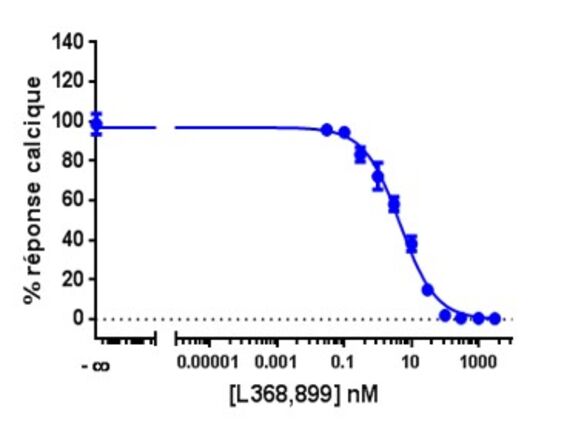
Calcium is involved in many physiological processes (neurotransmitter release, muscle contraction, blood coagulation...) and in cell signalling where it acts as a second messenger following the activation of heterotrimeric Gq proteins via G protein coupled receptors.
This test allows dynamic monitoring of variations in intracytoplasmic calcium concentration in living cells.