Introduction
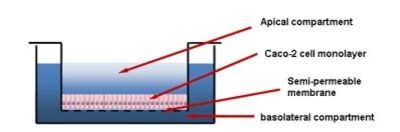
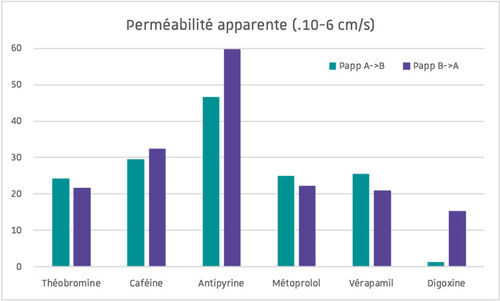
The ability of a molecule to cross the intestinal barrier is one of the properties studied in the ADME-Tox study of a compound.
The Caco-2 cell line (derived from a human colorectal carcinoma) is the model used in vitro to assess the absorption of a compound through the intestinal barrier.