Biodistribution is the study of the becoming of a drug candidate in an organism by the analysis of the evolution of its concentration in the targeted organs during time. The biodistribution analysis depends on the physiochimical characteristics of a drug candidate, on the blood absorbation, on the vascularisaton of the target, on its distribution and on its retention in the organs and all the biological barriers (blood-brain barrie, vascular wall…)
Biodistribution of a compound from the blood toward the targeted tissues or toward other tissues or organs, can bring to the apparition of indesirable effects, which can be measured at the animal or in pathological models.
It is indeed demonstrated that a phatology can modify the integrity and/or permeability of the biological barriers and can influence the ADME of a compound (Absorption, Bistribution, Metabolisation, Elimination)
Exemples :
-a local inflammation will increase the permeability of the blood capillaries and will also increase the disponibility of a compound in the inflammatory site.
- A tumor in an organ will also be more accessible due to the increase of vascularization.
-The passage of the Blood brain barrier (BBB) is also strongly influenced by the presence of a pathologie
-A touch with the renal or hepatic function can reduce the elimination of a drug candidate and increase its biological efficacity and/or its indesirable effects…
Determinating the biodistribution of a compound in a pathological compound is very utile.
There is no standard model that can prejudge of this biodistribution in the studied pathology. The biodistribution is essential to realise in the same model that the one used for the study of therapeutic efficacity of the drug candidate.
This model can be reproduced but it generaly demands a heavy focus and an external formation to validate the needed skills.
Because of these reasons, we propose to realise the biodistribution in your models, mastered and realized by your scientifical teams.
It is then possible to :
-make the transfers between the animals toward our service of zootechnics
-allow shifts in your establishment so we can realise the samplings in better conditions and according to our standards.
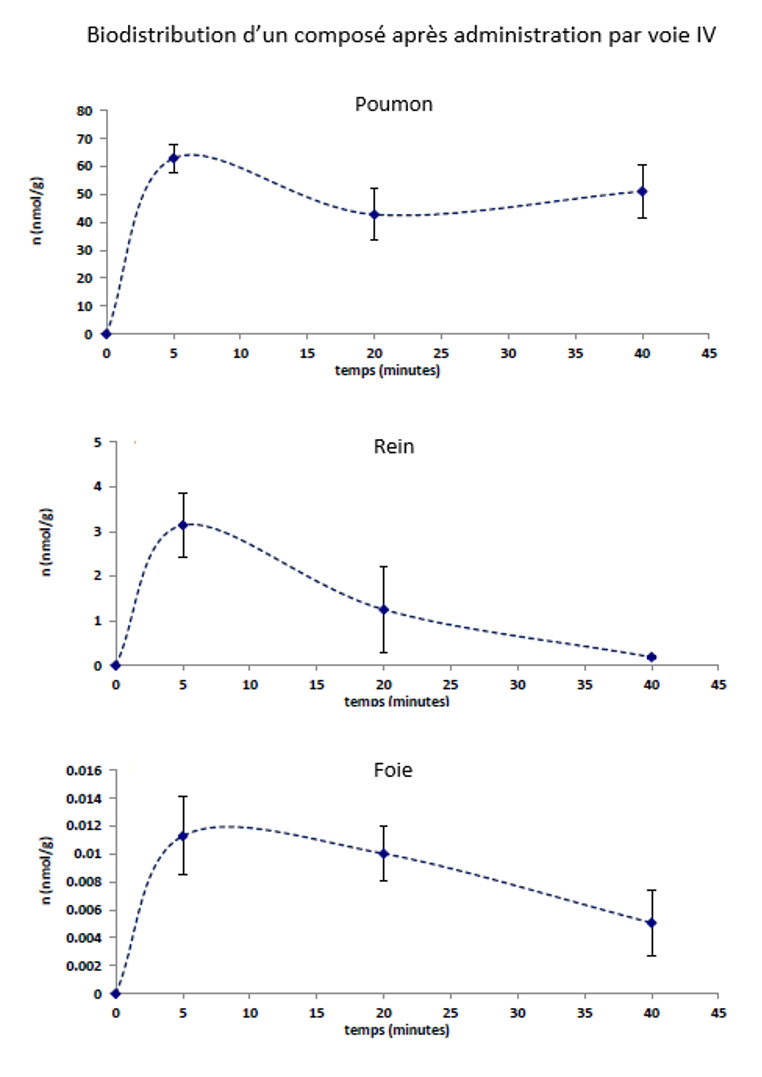
The biodistribution of a drug determines its therapeutic efficacity, its secondary effects and its toxicity.
The settings measured during a study of biodistribution :
-Maximal concentration of the principal actif in the interested organ(s) (C max)
-Necessery time for the principal actif to reach the concentration C max (T max)
-Half-life (time for maximal concentration to decrease of 50%)
It is also possible to study the metabolites when they have been previously identified.