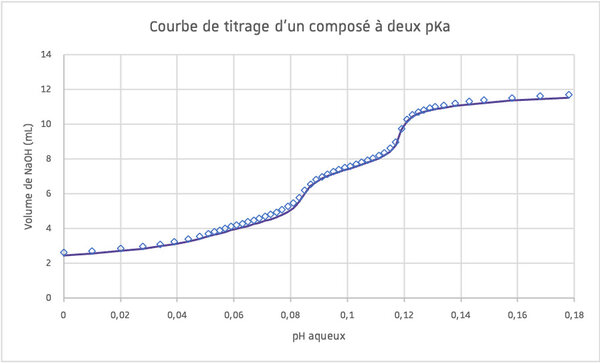
The pKa or equilibrium constant is the parameter measuring the strength of acids and bases. It gives the ionization state of a molecule at a given pH value.
The physical, chemical and biological properties of molecules depend on the state of ionization.
The vast majority of drugs contain ionizable groups.
Data are obtained by pH-metric titration.
Protocol
| Matrix | Water, constant ionic strength (I=0.1M) |
| Test temperture | 25°C |
| Quantification | pH-measurement |
| Data delivery | pKa |
| Replicat | 1 |
The compound is dissolved in water with excess strong acid and titrated with NaOH.
The pKa is obtained by fitting the titration curve.
Data
Notes
For hydrophobic compounds, different proportions of methanol are used in order to solubilize the compound. The aqueous pKa is obtained by extrapolation.